SERVICES
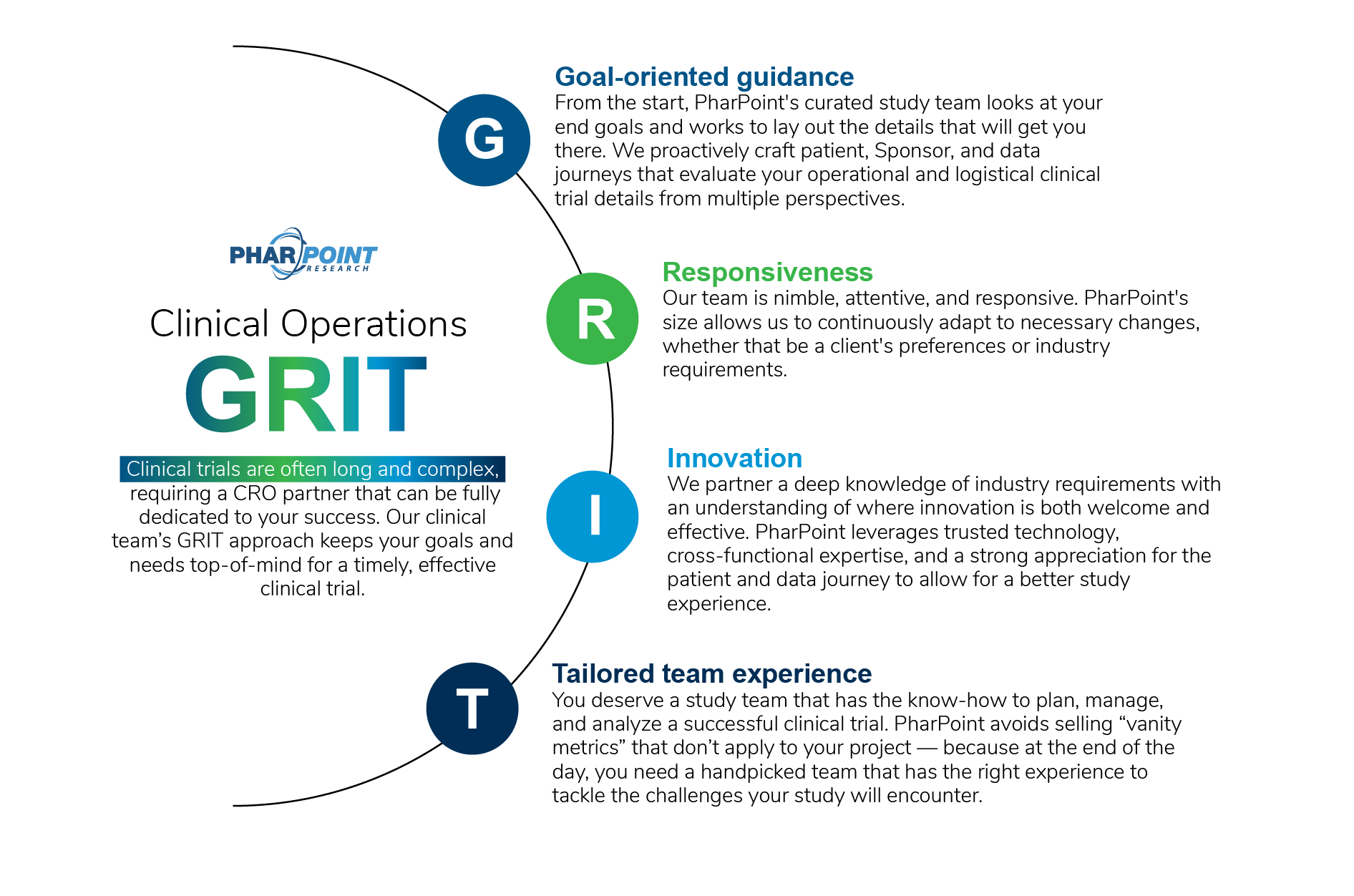
Clinical Operations and Project Management Services

PharPoint’s team of clinical research associates (CRAs), clinical operations leads (COLs), project managers (PMs), and other clinical staff are experienced and dedicated to timely, thorough support for your study sites.
We work to ensure study excellence and transparent collaboration between CRO, site, and Sponsor. Our teams are highly accessible and work closely to build excellent site relationships that streamline communications, encourage efficiency, and ensure compliance.
Our comprehensive clinical operations services include:
- Clinical consulting
- Risk mitigation
- Vendor management
- Strategic site selection
- Study start-up support, including investigator grant and research budget preparation
- Clinical monitoring services, including remote monitoring capabilities
- Clinical trial project management
- Study rescue
“Not only were documents provided to our site in a timely manner, communication with all parties from the CRA to the project managers has been excellent. When follow up is required, I can count on it being addressed quickly and accurately.”
STUDY COORDINATOR, SITE TESTIMONIAL
How can you increase speed to first patient in and maximize enrollment potential?
Download PharPoint’s Site Feasibility Whitepaper, which covers topics such as best practices for site selection and how to leverage Big Data, experience, and niche expertise to achieve your enrollment goals. Fill out the form below for access.
Mitigate risk & overcome study challenges
Every study has it’s own unique challenges, and the PharPoint team excels in identifying and addressing these obstacles before they wreak havoc on your study.
The extensive experience of our team, including collaboration from our biometric teams and other important stakeholders, gives us the right background to begin this planning.
From there, we’re able to make strategic recommendations and plans of action to allow you to reach study goals that matter most to you – within budget, and on time.
The Right Experience
Matched to the details of your study
A knowledgeable and attentive team is everything.
PharPoint strategically selects CRAs, COLs, and PMs according to each Sponsor’s individual study needs. Additionally, our culture of mentorship, training and development means each team member has the collective background of their colleagues to back strategic recommendations.
Therapeutic expertise:
Our CRAs, COLs, and PMs have diverse backgrounds. We work to align our subject matter experts (SMEs) by therapeutic expertise and availability for each study.
All COLs have CRA experience:
On-site activities are tracked and managed by the PM and/or COL. Regular performance assessment visits for CRAs are also completed by COLs (or functional managers).
Regional resources:
To decrease travel costs, we always strive to match regionally located CRAs to study sites.
Learn more about how our clinical research experts can help your current or upcoming study.
Set up time to speak with our business development team to learn the specifics of our clinical operations experience and team expertise.

