CAPABILITIES
Global CRO Service Solutions
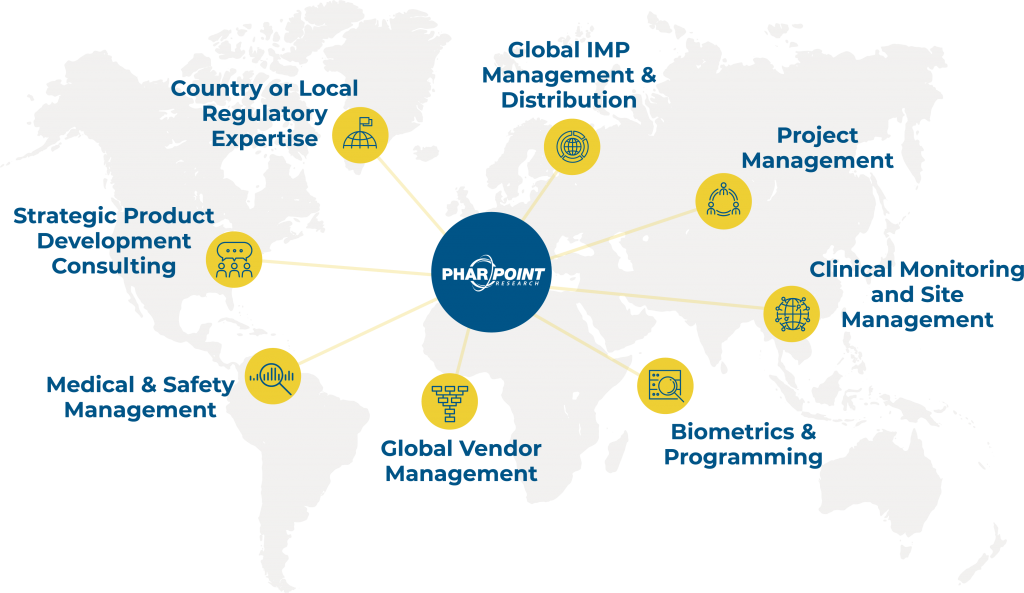
Country & regional specific knowledge and resources
For global studies, it is imperative that the study team have as much country and regional specific knowledge and resources to navigate the complicated regulatory environment unique to studies in multiple countries.
PharPoint has vetted global CRO partners in Europe, Latin America, and Asia Pacific to allow us to choose the right partner based on study needs. These resources would provide the regulatory and country specific knowledge needed but would report into the same project management structure as the North American based monitoring team to ensure consistent monitoring and clinical trial management.
Coordinated global efforts
PharPoint will work with our global partners to provide strategic planning and regional expertise for recommendation for country mix and navigation of regional regulatory requirements. PharPoint will coordinate the global team’s efforts to optimize regulatory approvals, ethic committee submissions/approvals, enrollment, and proactively manage obstacles such as subject screen challenges and excessive dropouts at the study, region, and site level.
Work with PharPoint's flexible, award-winning team.
PharPoint Research is an award-winning and client-focused contract research organization that has supported over 900 clinical trials since 2007 with an exceptional client retention rate. We’re able to offer sponsors global CRO support, with services including clinical operations, project management, data management, biostatistics, statistical programming, and strategic clinical trial consulting. For more information about our study specific expertise and how we can support your upcoming clinical trial, please contact our business development team.
