Identify and manage high-performing sites and get study results 30 days faster than the industry average with support from PharPoint’s dermatology-experienced team.
“High priority pivotal studies locked and delivered faster than any other CRO I have worked with in my experience. Not only was the study delivered within budget and early, it was a quality deliverable.”
CLIENT TESTIMONIAL
“There is an inherent honesty and authenticity in [PharPoint’s] approach. …I have complete faith and trust in PharPoint as our partner, and that gives me confidence in the success of our clinical program.”
CLIENT TESTIMONIAL
MEET AN EXPERT
PharPoint’s team provides attentive dermatology CRO services with the right experience & capabilities to support your trial.
Meet Denise, Senior Clinical Project Manager.
Denise has 24+ years of experience in clinical research, during which she’s supported numerous dermatology protocols, including large, global dermatology studies.
Experience is everything. PharPoint’s senior leadership team averages over 25 years in the industry. PharPoint has supported dermatology studies for 10+ years, including Phase 1 through Phase 4. Our teammates have experience supporting dermatology studies ranging from skin infections to aesthetic dermatology indications.
DERMATOLOGY SUCCESS STORY
Enrollment goals met five months early
PharPoint provided clinical and biometrics support for a US-based Phase 2 dermatology study, ultimately meeting enrollment goals five months early – despite closures due to COVID-19.
PharPoint worked collaboratively with Sponsor, sites, and other key study partners to allow the study to progress efficiently and enroll rapidly. A specialized patient recruitment partner was brought on, providing additional enrollment support to study sites. PharPoint’s attentive, supportive site relationships kept us – and the study Sponsor – in front-row seats to study progress, closely monitoring and responding to missed visits.
The result? A study that overcame difficult challenges and managed to enroll 5 months ahead of schedule.

Have confidence in your study’s ability to enroll on-time
Identify and activate the perfect sites:
PharPoint uses big data & CTMS alongside our growing internal investigator database to pinpoint dermatology sites with proven success. As part of our identification and analysis process, we provide Sponsors with a heatmap that consists of potential North American site locations and prospective available patient populations.
Predict enrollment:
PharPoint’s proprietary Enrollment Index projects a site’s ability to enroll patients based on patient availability, site resources, and competing trials. Based on your needs, PharPoint can anticipate the number of sites you’ll need to meet enrollment goals, including customized site number and duration models.
Get study results faster
With PharPoint as your Dermatology CRO, you’ll experience our team’s industry-best biometrics timelines. PharPoint can lock a database as soon as five days after LPLV SDV – approximately one month faster than the industry average, with topline results to follow only two days after.

Dermatology CRO support to fit your needs, from study planning through trial results
Our team has experience with numerous dermatology indications, patient populations, and phases, including:
Acne
Alopecia
Atopic Dermatitis
Central Abdominal Bulging
Cutaneous Lupus Erythematosus
Epidermolysis Bullosa
Hair Loss
Herpes Labialis Infection
Lipoma
Localized Abdominal Contour Defects
Lower Limb and Foot Ulcers
Onychomycosis
Psoriasis
Rosacea
Scleroderma
Sjogren-Larsoon Syndrome
Subcutaneous Adipose Tissue Reduction
Submental Subcutaneous Fat
Urticaria
Vitiligo
Venous Leg Ulcers

Work with a team that prioritizes your study goals
PharPoint works diligently on behalf of and alongside your team for a seamless study experience.
Top-of-mind and always accessible:
Unlike big-box CROs that can leave smaller Sponsors feeling overlooked and forgotten, PharPoint looks at Sponsor-CRO relationships as a partnership, prioritizing your study data and goals. This includes a clear escalation path.
Cross-functional communication:
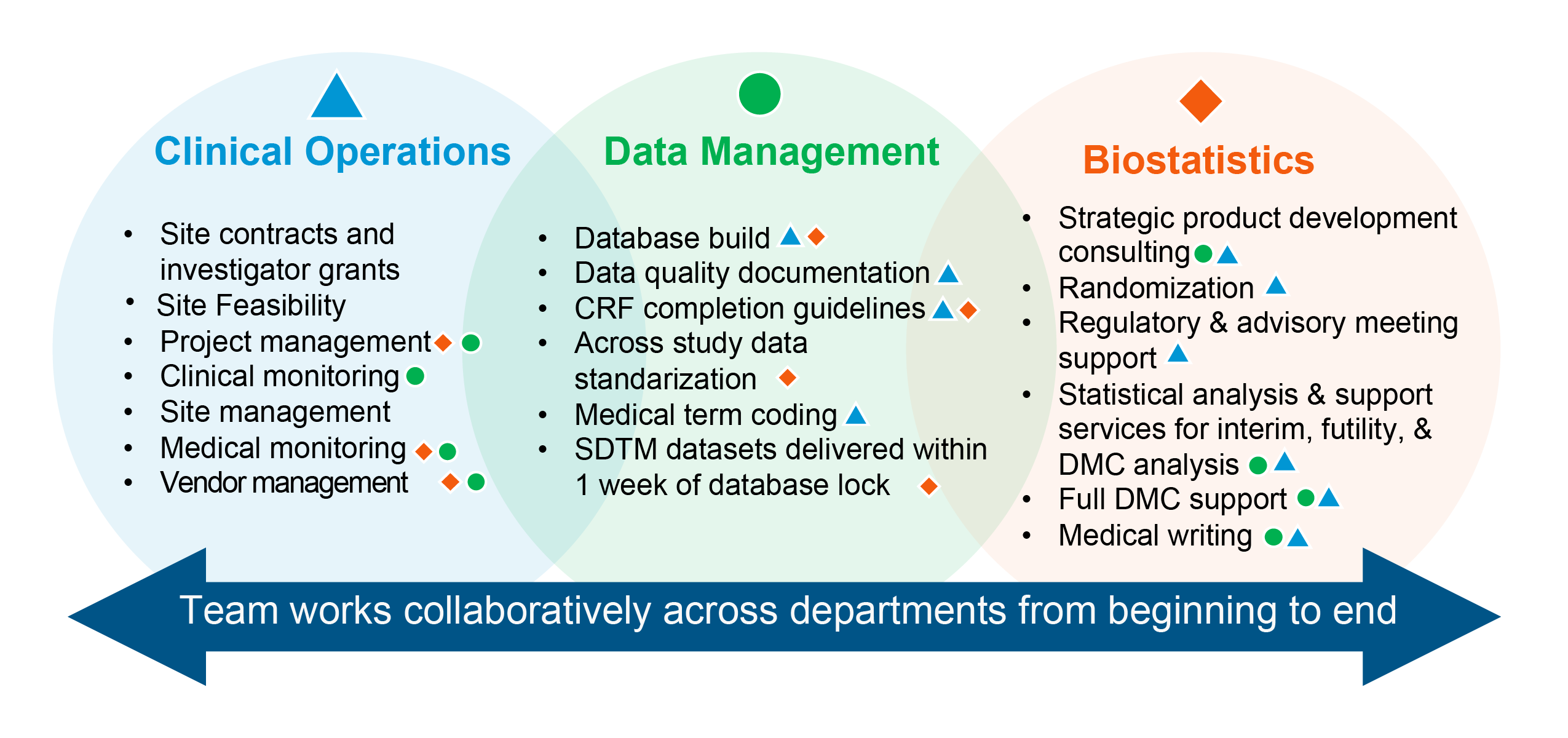
PharPoint’s study team works cross-functionally, avoiding silos. With different departments working together, expertise is shared, questions can be addressed promptly, and a trial’s many moving parts can work like a well-oiled machine.
Explore Additional Resources
Partner with our experienced team