CLINICAL TRIAL SERVICES
With PharPoint as your clinical data management CRO, your team gets study results faster.
Thanks to a seasoned team, efficient processes, and excellent communication, our clinical data management teammates are able to help clients expedite timelines and meet study goals.

DATABASE BUILD
PharPoint can build and release custom databases in 30 business days (compared to the industry standard of 68 days). PharPoint is proud to provide clients with custom deliverables – other CROs often push back on building custom forms or require an additional charge.
MID-STUDY CHANGES
According to a report from Tufts CSDD, the average clinical trial has 4 unplanned and 4 planned mid-study changes, each requiring about 30 days to make. At PharPoint, we repeatedly make small changes within 1-2 business days, with more complex changes taking 1-2 weeks.

DATABASE LOCK
Once last patient, last visit has occurred and final source data verification is complete, the PharPoint team locks a database within 5 business days, allowing sponsors to get study results about 30 days faster than industry average.

PharPoint’s clinical data management CRO services include:
- Study oversight and flexible clinical data management consulting support
- Study tools development
- Comprehensive data management plans
- Electronic data capture (EDC) platform selection
- Database development, validation, and testing
- Medical data coding
- Global data entry, cleaning, and verification
- Data import and data export specification preparation
- Data processing for paper and EDC studies
- Data standardization
FIVE DAYS TO DATABASE LOCK
How does PharPoint help clients achieve an expedited database lock?
EXPLORE RELATED RESOURCES
A U.S.-based data management CRO team with global capabilities
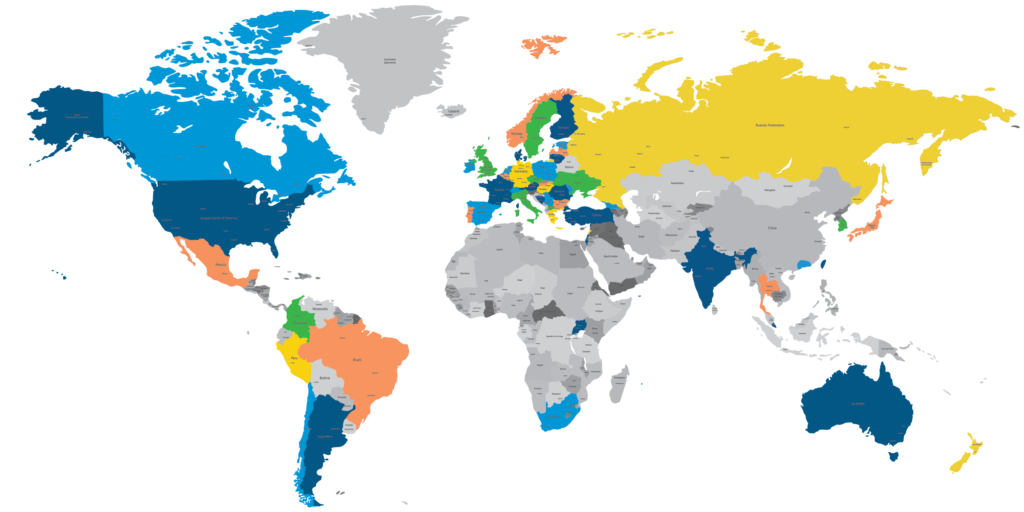
The PharPoint team has employees who work in our Research Triangle Park and Wilmington, North Carolina offices and remotely across the United States. While offshoring biometrics work is common practice at many CROs, none of PharPoint’s work is offshored, allowing us to maintain control over business functions and operations.

