BUILT FOR COLLABORATION
Together, we help you meet clinical milestones.
PharPoint is a contract research organization (CRO) that works collaboratively alongside likeminded sponsors and study partners to keep deliverable timelines clear, trial data current, and study goals in focus. Our cross-functional alignment and planning removes the silos and last-minute scrambles that can slow down clinical trial timelines and put participant health at risk – helping you meet clinical milestones with confidence.

RAPID BIOMETRICS TIMELINES
We repeatedly deliver industry-best end-of-study timelines.
“Fast timelines” are easy for CROs to promise and much harder to achieve. At PharPoint, our data management and biostatistics teams consistently deliver according to the standard timelines we promise. This consistent performance reflects a team that plans ahead, communicates openly, and follows through.
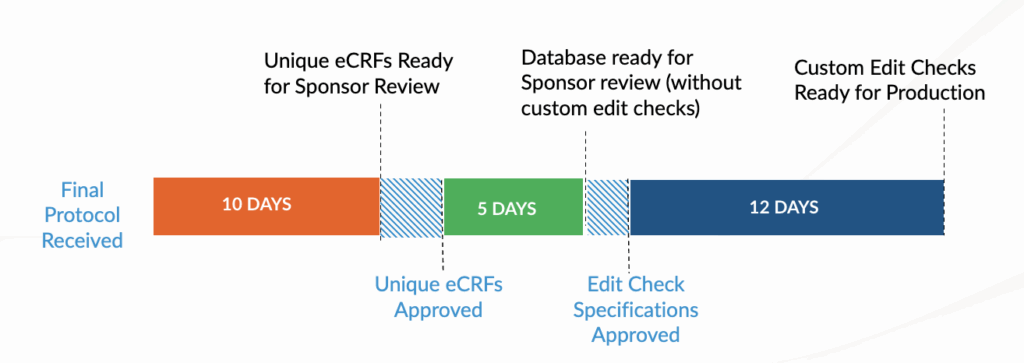
PharPoint’s data management team includes in-house database builders with experience across multiple EDC systems. Our standard timeline includes:
5 days
from last patient, last visit (LPLV) and final source data verification (SDV)
We know topline results are highly anticipated, and our team works diligently to deliver these results quickly. PharPoint’s standard timeline for topline results is 2 business days after database lock.
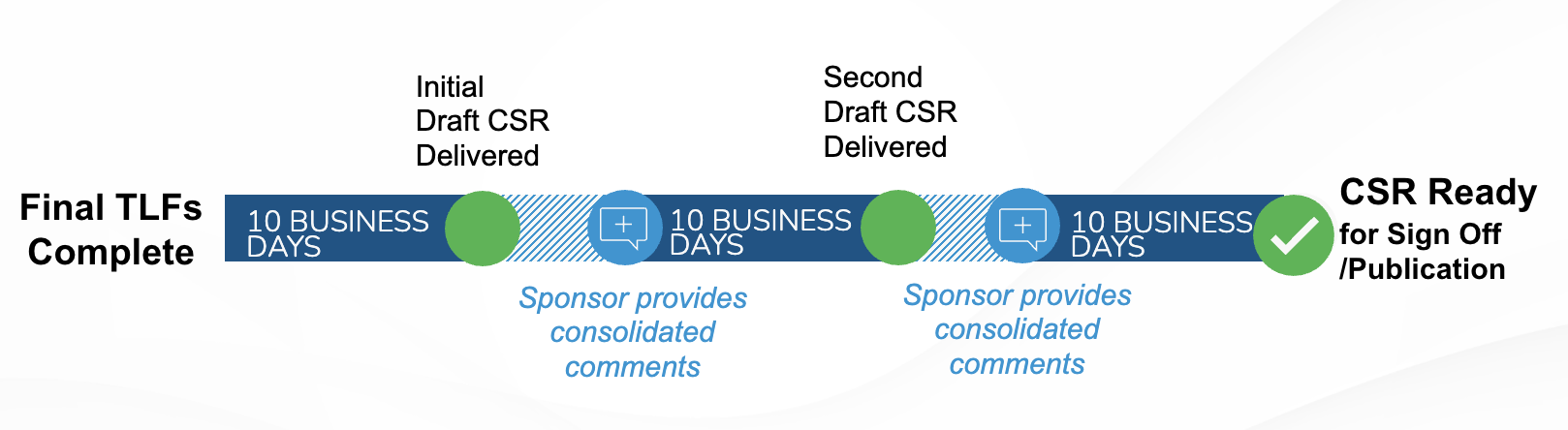
PharPoint’s standard timeline for the delivery of draft TLFs is 5 business days after database lock.
Final TLFs are delivered 5 more days after the receipt of draft TLF comments from the sponsor.
PharPoint’s standard CSR delivery timeline is 40 business days, assuming two rounds of 5-day sponsor review.
WHAT OUR CLIENTS SAY
What sets PharPoint apart from other CROs?
Our collaboration
Our team works cross-functionally to continuously align priorities, anticipate challenges and proactively provide solutions– allowing for better processes, stronger execution, and built-in quality by design.
Our transparency
Transparency and good business ethics are at the heart of everything we do: from the proposal process to how we communicate on-going study progress.
Our timelines
With strategic early planning and collaboration, efficient study processes, and a seasoned study team, PharPoint helps sponsors meet rapid study timelines.
ARTICLES & INSIGHTS