Supporting Blood Cancer Clinical Trials: Predicting Study Challenges
Treatment of blood cancer has dramatically improved over the last several decades. Innovators continue to make improvements and advancements through clinical research, with over 18,000 clinical trials spanning numerous hematologic cancers listed on clinicaltrial.gov as of August 2022.
What challenges should Sponsors conducting these trials anticipate, and how does the team at PharPoint Research – a CRO with oncology expertise – tackle these challenges?
Blood Cancer 101
Let’s start with the basics: what is blood cancer? The National Cancer Institute defines blood cancer, also called hematologic cancer, as cancer that begins in blood-forming tissue.
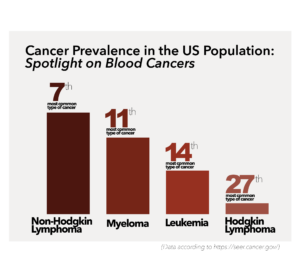
There are three primary types of blood cancer – leukemia (cancer of white blood cells), lymphoma (cancer of the lymphatic system), and myeloma (cancer of plasma cells).
According to Yale Medicine, blood cancers account for about 10 percent of all diagnosed cancers in the United States annually.
Addressing Common Obstacles in Blood Cancer Clinical Trials
Three of the most common obstacles our team sees within blood cancer clinical trials are high logistical complexity, low patient awareness, and the need for increased patient safety monitoring.
High Logistical Complexity
Blood cancer trials are logistically complicated. Any team working on a study must ensure the many moving parts align seamlessly.
How does PharPoint overcome this?
When it comes to managing complex trials seamlessly, experience is vital. The PharPoint team has experience in cohort management along with experience managing complicated logistics and is capable of mitigating risk and avoiding delays.
Low Patient Awareness
The patient population for leukemia/lymphoma is typically highly motivated, despite trial complexities. Despite this motivation, patient awareness is still a significant enrollment challenge.
How does PharPoint overcome this?
PharPoint’s team has relationships with numerous industry-leading sites across the United States and has experience engaging advocacy groups to increase patient awareness. These combined relationships and resources help to ensure institutions themselves are prioritizing your study and getting your trial in front of suitable patients.
Increased Patient Safety Monitoring
Safety monitoring in oncology frequently requires a much higher level of review and understanding due to the sheer volume of adverse experiences that must be characterized and documented.
How does PharPoint overcome this?
Our network of monitors is equipped with a preexisting comprehension of the CTCAE grading associated with toxicity management. They understand the impact of the interpretation of results on overall safety reporting and data analysis.