CLINICAL TRIAL SOLUTIONS
How can we support you?
Explore our Biometrics CRO Services
The PharPoint team works with sponsors of all size to provide high quality biometrics CRO services. Learn more about how our biometrics teams can help support your needs.
THE PHARPOINT DIFFERENCE
How can our team support your biometrics CRO needs?
AVOID HIDDEN COSTS
with Custom Programming
PharPoint’s statisticians use custom programming, transferring ownership to clients at the end of a study without any added costs. Other biometrics CROs often use proprietary macros for their programs, which can lead to problems for sponsors during the submission process.
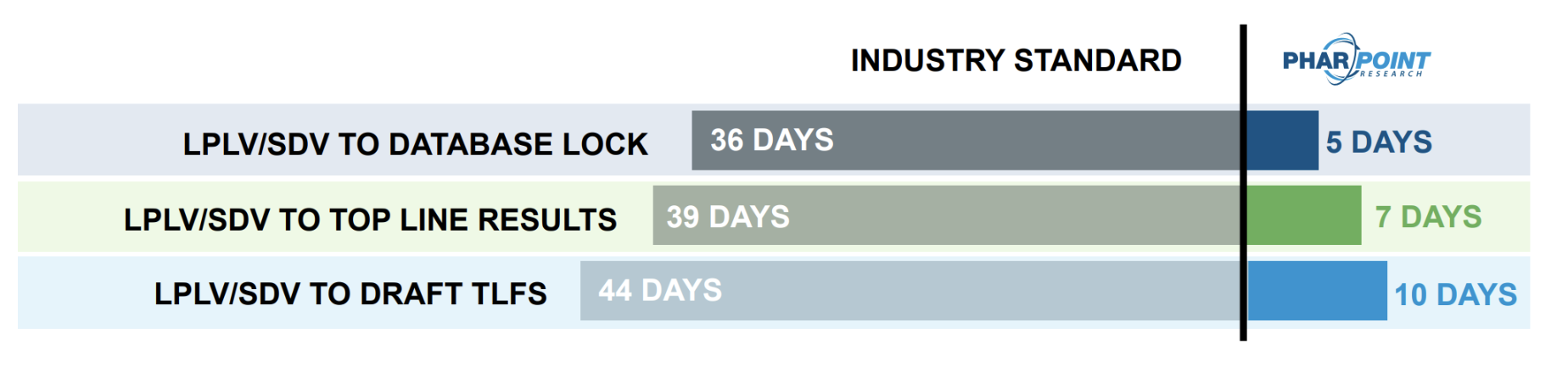
Get results faster
with Industry-best biometrics timelines
At every step, PharPoint’s standard biometrics CRO timelines are industry-best. Throughout your study, clean data are soft locked, reducing the number of subjects needing to be locked following Last Participant, Last Visit (LPLV) and decreasing the timeline to databse lock to only 5 days after final Source Document Verification (SDV).
CONFIDENTLY NAVIGATE REGULATORY DISCUSSIONS
with An Experienced Biometrics CRO Team
PharPoint biostatisticians can support discussion with regulatory authorities, with experience providing statistical representation for Type A, B, and C meetings.
SAVE TIME AND MONEY
with PharPoint’s Technology Partnerships
The PharPoint team has held partnerships with EDC providers Medidata and Medrio since 2011, including in-house certified study builders and administrators. These partnerships allow us to pass on cost and timeline advantages to our clients.
Related Resources

Solving Data Management Challenges with Strategic CRO Oversight

Achieving Expedited Database Lock with Director of DM, Wendy Moffett

A Guide to Reviewing Regulatory Documents as a Subject Matter Expert (SME)

Four Signs It is Time to Break Up with Your Regulatory Medical Writing Vendor

Standard Clinical Trial Timelines: A Sponsor’s Guide to Evaluating Biometrics CROs

