CLINICAL TRIAL SOLUTIONS
How can we support you?
Explore our Phase I CRO Services
PharPoint works diligently to help clients plan, manage, and analyze effective Phase I clinical trials. Our team has supported over 400 Phase 1 clinical trials and is a trusted partner for sponsors looking for full service Phase I CRO support.
THE PHARPOINT DIFFERENCE
Helping sponsors meet their Phase I study goals
AN EXPERIENCED TEAM
For Phase I Success
PharPoint aligns sponsors with teammates that have the right knowledge and expereince to overcome potential study challenges. Our Phase I CRO experience includes supporting first-in-human studies as well both studies that use normal healthy volunteers and patient populations.
IDENTIFY THE RIGHT SITES
with PharPoint’s Site Agnostic Approach
Finding the right sites to support your trial is a vital step in ensuring your study will enroll on time. Rather than pushing clients toward owned units that may not fit study needs, PharPoint uses a site-agnostic approach.
We prioritize identifying and selecting the best-possible site parter for your unique study details and requirements. This process evaluates factors such as availability, competing studies, patient access, and unique capabilities and experience – ultimately matching clients with site partners that will help them meet study goals.
EXPEDITE SITE ACTIVATIONS
with Our Pre-Qualified Network
PharPoint’s expanding site relationships includes a number of pre-qualified US sites with CDAs in place to expedite site activation. These sites have strong clinical trial capabilities that are matched to specific study needs as part of our site-agnostic approach.
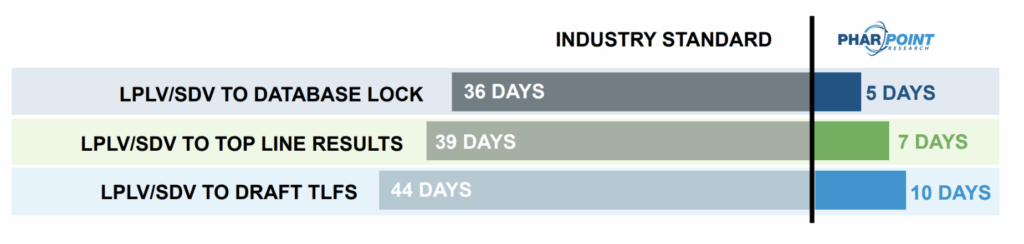
GET RESULTS FASTER
with PharPoint’s Industry-Best Biometrics Timelines
At every step, PharPoint’s standard biometrics CRO timelines are industry-best. Throughout your study, clean data are soft locked, reducing the number of subjects needing to be locked following Last Participant, Last Visit (LPLV) and decreasing the timeline to database lock to only 5 days after final Source Document Verification (SDV).
Related Resources

CRO Study Team Continuity and Transparency

No Surprises: PharPoint’s Transparent Approach to Changes in Scope

Phase 1 CRO Project Management: Avoiding Delays

Building Stronger Relationships between CROs and Small Biopharma: Ensuring Your Voice is Heard

Exploring Benefits, Eligibility, and Key Considerations for Sponsors Pursuing Orphan Drug Designation

