CLINICAL TRIAL SOLUTIONS
How can we support you?
Explore our Phase II-III CRO Services
PharPoint works diligently to help clients plan, manage, and analyze effective Phase II-III clinical trials. Whether you are looking for individual service support or looking to fully outsource your trial operations, our team is happy to chat.
THE PHARPOINT DIFFERENCE
Helping sponsors meet their Phase II and Phase III study goals
AN EXPERIENCED TEAM
For Phase II-III Success
PharPoint aligns sponsors with teammates that have the right knowledge and experience to proactively identify factors critical to study success and mitigate potential study challenges. Working with the PharPoint team also means clear communication pathways and access to senior resources.
WORK COLLABORATIVELY
with a team that works without silos
From the start, the PharPoint team works collaboratively across functions to ensure study decisions are made with input from multiple perspectives. This connected approach helps us better anticipate challenges, accelerate timelines, and support deliverables that have our clients end goals in mind.
GET RESULTS FASTER
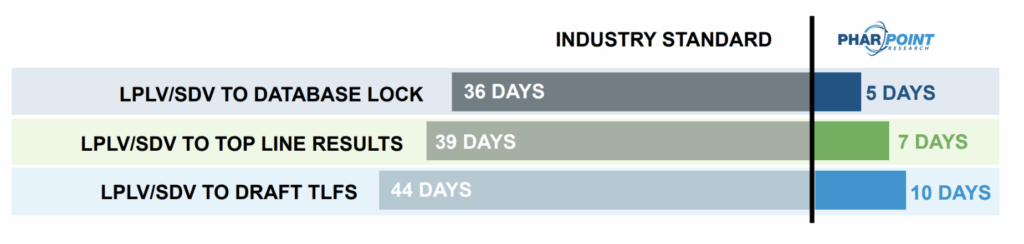
with PharPoint’s Industry-Best Biometrics Timelines
At every step, PharPoint’s standard biometrics CRO timelines are industry-best. Throughout your study, clean data are soft locked, reducing the number of subjects needing to be locked following Last Participant, Last Visit (LPLV) and decreasing the timeline to database lock to only 5 days after final Source Document Verification (SDV).
Explore Related Resources

Solving Data Management Challenges with Strategic CRO Oversight

Achieving Expedited Database Lock with Director of DM, Wendy Moffett

A Guide to Reviewing Regulatory Documents as a Subject Matter Expert (SME)

Four Signs It is Time to Break Up with Your Regulatory Medical Writing Vendor

Standard Clinical Trial Timelines: A Sponsor’s Guide to Evaluating Biometrics CROs

