As sponsors plan clinical trials, known risks to clinical trial budgets are often put under a microscope: Can my study meet enrollment goals? Will my outsourced vendors be able to deliver according to proposed goals? Do I have a clear regulatory strategy?
Another potential underestimated clinical trial budget risk is the protocol amendment.
While amendments are often necessary, they are also, to an extent, preventable. When preventable amendments show up in your trial, they can quietly drain hundreds of thousands of dollars from your budget.
How Common are Protocol Amendments?
Within industry-sponsored trials, a Cochrane review of studies comparing a trial’s initial plan to their final report showed that they are common, yet underreported.
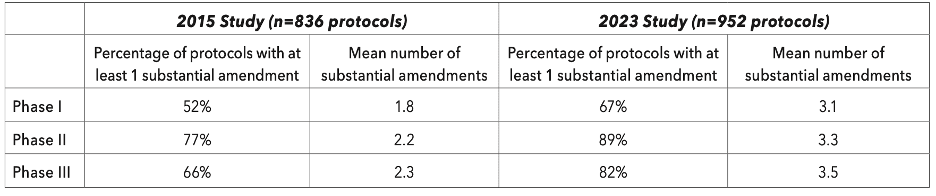
Over half of trials submitted at least one amendment, with changes most commonly being made to trial population (e.g, eligibility criteria). The frequency and number of amendments per study has increased across the board from 2015 to 2023 (see Table 1.0 below).
Table 1.0: Trends in Protocol Amendment Experience (via Tufts CSDD)
For researchers outside of industry, a separate analysis showed that numbers from non-commercially funded trials might be similar, with 53% of NHS-sponsored trials having at least one amendment, and the mean number of amendments sitting at 4.5.
Which Trials are Most Likely to Have Amendments?
Perhaps unsurprisingly, data from Tufts CSDD suggests that protocol amendments are highly associated with larger more complex studies. This is important to note, as study complexity is on the rise, with more trials targeting more specific patient populations and an increasing popularity of trials spanning multiple countries around the globe.
When looking at specific types of trials, protocol amendments were noted to be higher in both prevalence and frequency for:
- Oncology trials vs. non-oncology trials
- Large molecules vs. small molecules or vaccines
Why do Amendments Happen?
In some instances, protocol amendments are unavoidable. This may include scenarios where there have been specific regulatory agency requests related to the study or changes due to the development of new safety information.
However, Getz et al. had previously suggested that somewhere between 33-45% of amendments are avoidable.
These avoidable amendments might be caused by things like:
- Flaws in trial design
- Inconsistencies or lack of clarity in the protocol
- Unfeasible eligibility criteria, and
- Recruitment challenges
The Impact of an Amendment
How do protocol amendments impact clinical trials? Amendments incur significant expense for sponsors, delay trial timelines, and incur unexpected burden for investigative sites participating in the trial.
Timeline Delays
Amendments may delay timelines by 3-6 months. These delays stem largely from the regulatory and operational steps required to implement changes.
Any changes to a clinical trial protocol must be reviewed and approved by an independent review board (IRB) or ethics committee (IEC).
The time it takes for IRBs to review amendments depends on both the complexity of the changes and the IRB’s workload. Minor amendments might be reviewed within a few weeks. However, significant amendments (e.g., changes to study design, an increase in drug dosage, a significant increase in the number of subjects, the addition or elimination of a test intended to monitor safety, the addition of a new investigator) can take much longer. According to Tufts CSDD, the average time from internal approval of a substantial amendment to final ethics committee approval in 2023 was approximately 260 days. Notably, the first patients were reconsented after just 89 days, suggesting that sites may operate under different protocol versions for over seven months.
Tufts also found that trials with at least one substantial amendment experienced nearly three times longer durations from First Patient First Visit (FPFV) to Last Patient Last Visit (LPLV) compared to trials without such amendments.
Estimated Price of a Single Amendment
The average cost of a single amendment (excluding the general cost of timeline delays and impact to unrealized revenue) varies greatly, but is typically in the 6 digits. (See Table 2.0)
Table 2.0: A Breakdown of the Cost of a Single Amendment
| Activities impacted | Low estimate | High estimate |
| IRB/EC resubmission and updates | $20,000 | $50,000 |
| Site/vendor retraining and contracting | $50,000 | $100,000 |
| EDC reprogramming | $25,000 | $75,000 |
| Subject re-consenting and coordination | $10,000 | $25,000 |
| Project team time (PMs, CRAs, etc.) | $50,000 | $100,000 |
| Total estimated amendment cost | $160,000 | $350,000 |
Conclusion
Protocol amendments are a common yet often underestimated risk to clinical trial budgets and timelines. While some amendments are unavoidable, many stem from issues that could be identified and addressed during early protocol development.
By prioritizing feasibility and clarity from the outset, sponsors can reduce the likelihood of costly changes down the line.
If you’re looking to strengthen your protocol strategy and minimize amendment-related risks, consider reaching out to PharPoint Research for expert support in protocol feasibility and review.
Supporting Sources
Botto, E., Smith, Z., & Getz, K. (2024). New benchmarks on protocol amendment experience in oncology clinical trials. Therapeutic Innovation & Regulatory Science, 58, 645–654. (https://link.springer.com/article/10.1007/s43441-024-00629-2)
Chatsiricharoenkul, S. (n.d.). Protocol Amendment Decision: IRB’s Role. Nepal Health Research Council. Retrieved from https://elibrary.nhrc.gov.np/bitstream/20.500.14356/2543/1/Protocol%20Amendment%20Decision%20IRB%27s%20Role.pdf3
Dwan, K., Altman, D. G., Cresswell, L., Blundell, M., Gamble, C. L., & Williamson, P. R. (2011). Comparison of protocols and registry entries to published reports for randomised controlled trials. Cochrane Database of Systematic Reviews, 2011(1), MR000031. https://doi.org/10.1002/14651858.MR000031.pub2[1](https://www.cochrane.org/evidence/MR000031_comparison-protocols-and-registry-entries-published-reports-randomised-controlled-trials)
Getz, K., Smith, Z., Botto, E., Murphy, E., & Dauchy, A. (2024). New benchmarks on protocol amendment practices, trends and their impact on clinical trial performance. Therapeutic Innovation & Regulatory Science, 58(3), 539–548. PMID: 384386584
Getz, K. A., Zuckerman, R., Cropp, A. B., Hindle, A. L., Krauss, R., & Kaitin, K. I. (2011). Measuring the incidence, causes, and repercussions of protocol amendments. Therapeutic Innovation & Regulatory Science, 45(3), 265–275. (https://link.springer.com/article/10.1177/009286151104500307)
Joshi S. (2023). Common Clinical Trial Amendments, why they are submitted and how they can be avoided: a mixed methods study on NHS UK Sponsored Research (Amendments Assemble). Trials, 24(1), 10. https://doi.org/10.1186/s13063-022-06989-0
RELATED RESOURCES